
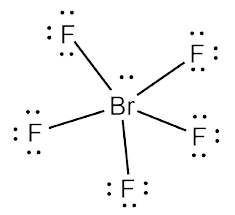
So the Hybridization of Brf5 molecules is sp 3d 2. Bromine atoms at this point are excited and their will occurrence of hybridization takes place.įour of the orbitals are 4s, three 4p, and two 4d, while the six steric nodes are involved in hybridization. There are two p-orbital that are unpaired. Some electrons are shifted to 4d-orbitals to obtain a pentavalency. Total valence electrons = 42 Brf5 hybridizationīromine atom Electronic configuration: 1s 2 2s 22p 6 3s 23p 63d 104s 24p 5. In total Brf5 consist of 42 valence electrons and is calculated as, There are seven valence electrons present on the bromine(Br) atoms and seven valence electrons on fluorine(F) atoms. These lone pairs of electrons are also called non-bonding electrons Brf5 valence electrons Hence, there is only one lone pair present in Bromine(Br) hybrid orbitals. Valence electron of Br = 7 and there are five sigma bonds present between bromine and fluorine atoms. Thus Center atom Bromine in Brf5 lewis structure has expanded its octet. Once knowing that, how many electrons are present in Brf5, the distribution of electrons around the central atom is done.
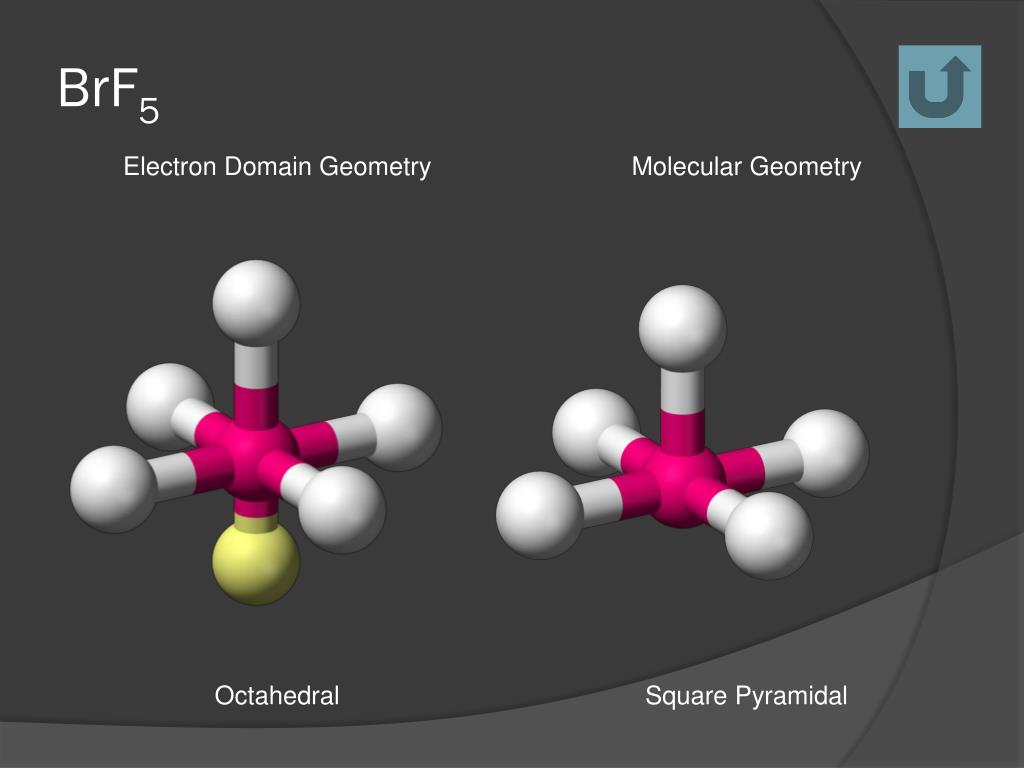
In the Brf5 lewis structure, bromine (Br) belongs to period four, which means it has a valence electron capacity greater than eight. This is known as the octet rule and is used to work out how close an atom is to a specific other atom or molecule. The square pyramidal effect of BrF5 molecular structures is due to the individual electron pairs on the core bromine atom.īrf5 lewis structure angle Brf5 lewis structure octet ruleĪ molecule needs eight electrons to complete its octet, which means they need 8 electrons in the outermost shell. It indicates that the basic atom has a single pair of electrons. Brf5 lewis structure shapeīrf5 has a pyramidal square shape. While the bromine (Br) atom is in the center, the fluorine (F) atoms are scattered around it.

The bromine (Br) atom makes up one of the five fluorine atoms in Brf5. There is no resonance observed in Brf5 and no isomers exist in Brf5. We can distribute the valence shell around the nucleus to fill the outermost layers of each atom once we know how many there are in Brf5. In Lewis structure of Brf5 is composed of 42 valence electrons. A Lewis dot structure consists of five Br-F bonds in Brf5. Thus total electron pairs for bonding = 21, and participate in bond formation.īromine act as a central atom due to less electronegativity than fluorine.


 0 kommentar(er)
0 kommentar(er)
